- Novelties in Regulation (EU) No 609/2013
- Food for infants and young children
- Foods for special medical purposes
- Foods intended for use in low-calorie diets for weight loss
- Food which is outside the scope of Regulation (EU) No 609/2013 and therefore without specific legislation.
- Notification of placing on the market
- Questions and answers on the application of Regulation (UE) No 609/2013
Foods for special medical purposes
Food for special medical purposes means foods specially processed or formulated and intended for the dietary management of patients to be used under medical supervision. It is intended for the exclusive or partial feeding of
patients with a limited, impaired or disturbed capacity to take, digest, absorb, metabolise or excrete ordinary food or certain nutrients contained therein, or metabolites, or with other medically-determined nutrient requirements, whose dietary management cannot be achieved by modification of the normal diet alone, with other food intended for a particular diet, or both.
How are they regulated?
Since 20 July 2016
The compositional and information requirements for food for special medical purposes are generally regulated by Regulation (EU) No 609/2013 and specifically by Commission Delegated Regulation (EU) 2016/128, of 25 September 2015, supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for food for special medical purposes.
Versión consolidada a fecha 15 de septiembre de 2017 del Reglamento Delegado (UE) 2016/128 de la Comisión de 25 de septiembre de 2015 que complementa el Reglamento (UE) n o 609/2013 del Parlamento Europeo y del Consejo en lo que respecta a los requisitos específicos de composición e información aplicables a los alimentos para usos médicos especiales (Texto pertinente a efectos del EEE)
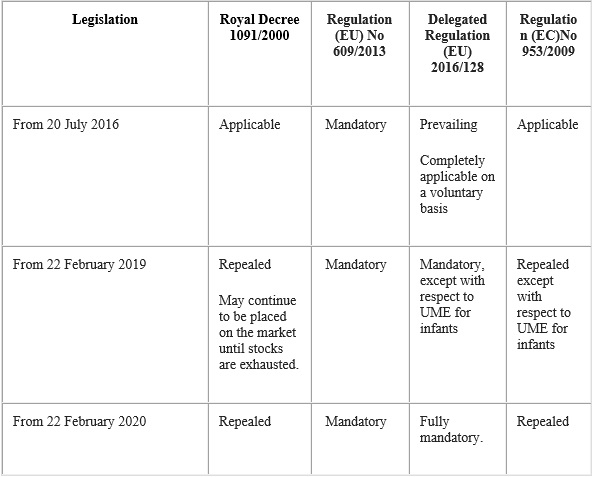
The provisions established in Regulation (EU) No 609/2013 are mandatory from 20 July 2016.
Delegated Regulation (EU) 2016/128 will be mandatory from 22 February 2019, except with regard to food for special medical purposes intended to satisfy the nutritional requirements of infants which shall be applicable from 22 February 2020. Up until these dates, Royal Decree 1091/2000, of 9 June will continue to be applicable. Nevertheless, operators who opt to adhere on a voluntary basis to the Delegated Regulation, may do so from 20 July 2016 provided they satisfy in full the requirements established in the same.
Nevertheless, operators who opt to adhere on a voluntary basis to Delegated Regulation (EU) 2016/128 prior to the dates of mandatory implementation may do so provided they satisfy in full the requirements established in the same.
In addition, article 3 of Regulation (EU) No 609/2013, establishes that to ensure the uniform implementation of this Regulation, the Commission may decide, by means of implementing acts wheterh a given food falls within the scope of this Regulation or to which specific category of food regulated in this REgulation a given food belongs.
The Commission asks the EFSA to provide a technical and scientific guide on food for special medical purposes in the context of article 3 of Regulation (EU) No 609/2013, for guidance in the preparation and presentation of well-structured dossiers to assess the extent to which a food product notified as food for special medical purposes falls under the scope of Regulation (EU) No 609/2013 under the proposed use.
The guide drafted by the EFSA in response to the Commission's request and published in November 2015 is available by clicking on the following link:
https://www.efsa.europa.eu/en/efsajournal/pub/4300
Table summarising the application of the transition periods of the legislation on food for special medical purposes:
Principal novelties of Delegated Regulation (EU) 2016/128:
- As regards the composition:
- minimum changes in the compositional requirements of food for medical purposes intended for infants in line with Delegated Regulation (EU) 2016/127.
- As regards labelling:
- The name of the food shall be “food for special medical purposes”.
- In Spanish, change of the mandatory indication “for the dietary treatment of …” to “for the dietary management of…” where the blank shall be filled in with the disease, disorder or medical condition for which the product is intended.
- Specific requirements are established on the nutritional declaration of the food for special medical purposes.
- Nutritional or health claims may not be made on food for special medical purposes.
- All the regulations on labelling, presentation and advertising applicable to formulae for healthy infants are extended to food for special medical purposes for infants.
- The implementation of requirements on pesticides in foods for special medical purposes intended for infants and young children is extended.
- As regards the notification procedure for placing on the market:
- This is maintained for food for special medical purposes.