- Novelties in Regulation (EU) No 609/2013
- Food for infants and young children
- Foods for special medical purposes
- Foods intended for use in low-calorie diets for weight loss
- Food which is outside the scope of Regulation (EU) No 609/2013 and therefore without specific legislation.
- Notification of placing on the market
- Questions and answers on the application of Regulation (UE) No 609/2013
Food for infants and young children
Infant formulae and follow-on formulae
Infant formulae and follow-on formulae are liquid foodstuffs intended to satisfy the nutritional requirements of healthy infants (children under the age of 12 months). When these formulae are prepared totally from protein from cows' milk or goats' milk, they may be called infant milk or follow-on milk.
Infant preparations are the only processed food products which satisfy by themselves the nutritional requirements of infants during the first months of life until the introduction of complementary feeding. Follow-on preparations are intended for infants when appropriate complementary feeding is introduced and they constitute the principal liquid element in the progressively diversified diet of such infants.
How are they regulated?
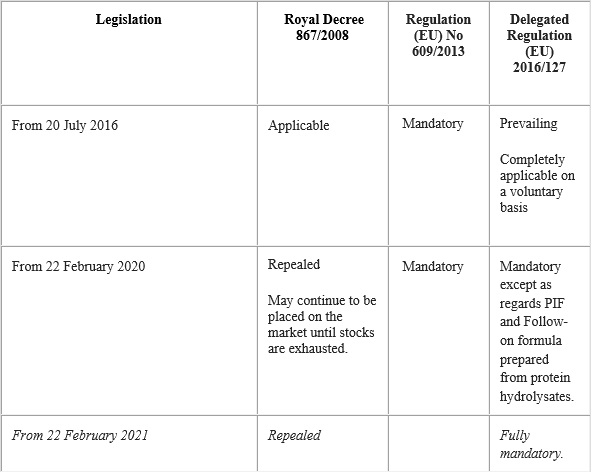
Since 20 July 2016
The compositional and information requirements for infant and follow-on formulae are generally regulated by Regulation (EU) No 609/2013 and specifically by the Commission Delegated Regulation (EU) 2016/127, of 25 September 2015, supplementing Regulation (EU) No 609/2013 of the European parliament and of the Council as regards the specific compositional and information requirements for infant and follow-on formulae.
The provisions established in Regulation (EU) No 609/2013 are mandatory from 20 July 2016.
In addition, as a novelty, Regulation (EU) No 609/2013, extends the ban on the use of pictures of infants or other pictures or text which may idealise the use of the products to follow-on formula, as up until 20 July 2016 this was only banned for infant formulae.
Delegated Regulation (EU) 2016/127 will be mandatory from 22 February 2020, except with regard to the infant formulae and follow-on formulae prepared from protein hydrolysates which shall be applicable from 22 February 2021. Up until these dates, Royal Decree 867/2008, of 23 May will continue to be applicable.
Nevertheless, operators who opt to adhere on a voluntary basis to Delegated Regulation (EU) 2016/127 prior to the dates of mandatory implementation may do so provided they satisfy in full the requirements established in the same.
Table summarising the application of the transition periods of the legislation on infant formulae and follow-on formulae:
Principal novelties of Delegated Regulation (EU) 2016/127:
- As regards the composition:
- the quantities of some macro and micronutrients have been increased or decreased in function of the EFSA ruling.
- The addition of DHA (Docosahexaenoic acid) is mandatory in all infant and follow-on formulae.
- The use of protein hydrolysates in infant formulae and follow-on formulae should be evaluated case-by-case. To date, only one formula containing partially hydrolysed whey protein has been positively evaluated so far by the EFSA, the specifications of which are listed in Annex I and II of the Delegated Regulation.
- As regards labelling:
- Specific requirements on how to provide the nutritional information for infant and follow-on formulae are established.
- Nutritional or health claims may not be made on infant formulae.
- The conditions of use of statements relating to lactose and DHA are regulated.
- As regards the notification procedure for placing on the market:
- This is maintained for infant formulae and, in certain cases it is extended to follow-on formulae manufactured from protein hydrolysates or follow-on formulae containing products other than those listed in annex II of the delegate Regulation.
Cereal-based foods and infant foods
Cereal-based food and baby food are foodstuffs intended to fulfil the particular requirements of infants (children under 12 months old) and young children (aged from 1 to 3 years) in good health, as a supplement to their diet and/or for their progressive adaptation to ordinary food. These include processes cereal-based food and purées of vegetables, fruit, meat or fish.
How are they regulated?
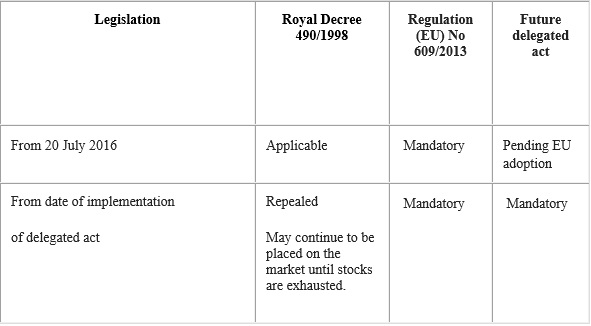
Since 20 July 2016
The compositional and information requirements for processed cereal-based food and baby food are generally regulated by Regulation (EU) No 609/2013.
In accordance with this Regulation, the European Commission must adopt a delegated act that specifically regulates the composition and information about these products, which is pending adoption. The Commission is to ask the EFSA for a revision of the compositional requirements of food for infants and young children in good health, intended for infants during weaning and young children, as a supplement to their diet and/or for their progressive adaptation to ordinary food. Based on the EFSA information, the Commission will propose a delegated act for adoption.
Therefore, the provisions established in Regulation (EU) No 609/2013 will be mandatory from 20 July 2016, and Royal Decree 490/1998, of 27 March 1998, and its amendment, remain in force until the date of implementation of the delegated act which is to regulate them in the future.
Table summarising the application of the transition periods of the legislation on processed cereal-based food and baby food