Removal of infant formulae manufactured in France
Updated 23 January 2018
On 11 December, the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN), received notification from the French Health Authorities of the withdrawal of infant formulae manufactured in France by the company Lactalis International, associated with an outbreak of Salmonellosis in infants under the age of 6 months in France.
In light of this information, the Agency contacted the responsible persons from the Rapid Alert System for Food and Feed (RASFF) and the French Authorities, and the company concerned, LactalisNutricion Iberia SLU, in order to obtain further information.
Consequently, Lactalis Nutricion Iberia SLU, decided to withdraw several batches of their infant formulae from the Spanish market, as a precautionary measure as these were manufactured on the same production line as the French made batches.
The company also reported that the consumption of the remaining products and batches made by Sanutri, Damira and Puleva are totally safe, including all the liquid milk products made by Puleva Peques, which are manufactured in factories belonging to the company in Spain.
As an additional precautionary measure, the company decided to shut down the factory in order to apply additional in-depth disinfection and cleaning measures.
On 11 December, at the last moment, the European Rapid Alert System for Food and Feed officially communicated the alert reported by the French Authorities giving notice of the batches involved, distributed at European Union level (France, Greece, Holland, Switzerland and Spain), and at third country level (Afghanistan, Bangladesh, Burkina Faso, Bahrain, the Congo, Ivory Coast, Cameroon, China, Algeria, Gabon, Georgia, China (Hong Kong), Iraq, Cambodia, Kuwait, Lebanon, Morocco, Madagascar, Mali, Peru, Pakistan, Paraguay, Qatar, Saudi Arabia, Sudan, Togo, Turkey, Taiwan, Ukraine, Vietnam, Yemen).
On 21 December, the Lactalis Nutricion Ibérica S.L. group informed this Agency that they were going to withdraw from the market all the products manufactured at the Craón plant (France), since 15 February, in application of the precautionary principle.
This new withdrawal affected a total of 30 batches which included SANUTRI and DAMIRA powdered infant formula and a powdered maltodextrin-based modular nutrition supplement.
On 17 January 2018 the French authorities reported that the company Lactalis International had decided to extend the withdrawal and recovery measures to all products manufactured at the Craón plant, regardless of the batch numbers and dates of manufacture. The company adopted this measure to facilitate the implementation of recovery and withdrawal measures, given the large number of batches involved.
The company is contacting all its customers to withdraw the new products from the market.
The batches in question can be found in the attached lists.
The Competent Authorities of the Autonomous Regions have been informed via the national Food Alert Network (SCIRI).
As a precautionary measure, any consumers who may have products from the batches given in the following list at home are advised to refrain from consuming them and to return them to the points of sale.
For further information, LACTALIS NUTRICIÓN IBERIA SLU has activated an information telephone number available for consumers should they have any queries: 900102336.
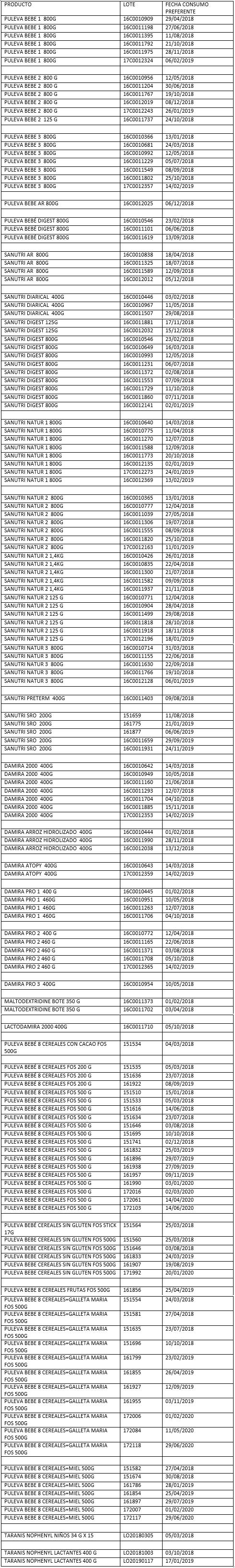
List of products affected on 11/12/2017
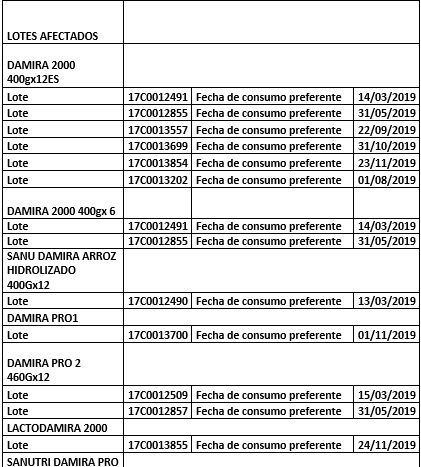
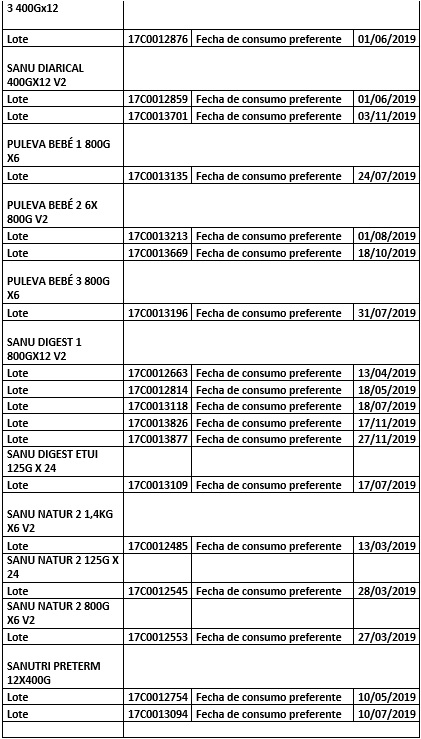
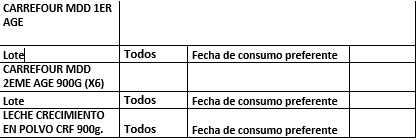
List of products affected on 22/12/2017
(en azul y con un Asterisco se marcan los lotes y productos que NO han sido distribuidos y están inmovilizados en el almacén)
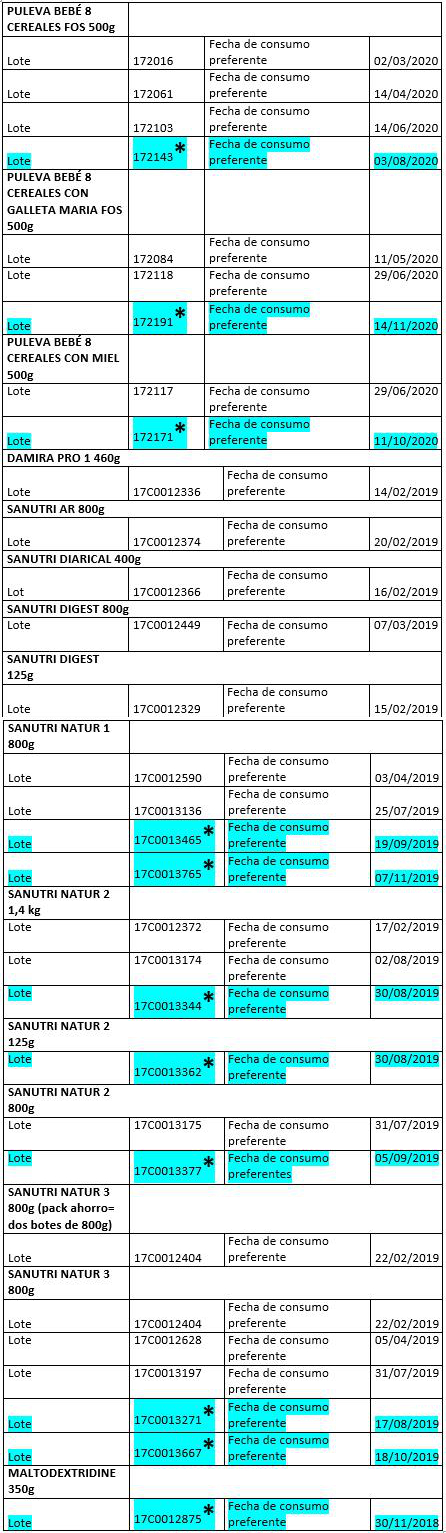
Listado de productos y lotes objeto de retirada a 17 de enero de 2018